|
Research Interests
The delicate balance between
the role of trace metals as micronutrients and toxicants
plays a crucial role in the maintenance of life in natural
systems. Trace metals exist in a variety of chemical forms
in the aquatic environment, ranging from free metal ions to
small inorganic complexes to large complexes with natural
organic matter (NOM) — each with its own unique properties.
The biological availability, and hence toxicity, of metals
in aquatic systems is strongly dependent on its chemical
speciation. Knowledge of the metal distribution among their
different physical and chemical forms (i.e. chemical
speciation) is therefore essential for predicting their fate
and environmental impacts.
Formed by the decomposition
of decaying plant and animal matter, NOM, such as humic and
fulvic acids, plays a key role in controlling chemical
speciation and metal bioavailability through the regulation
of free metal ion concentrations, which has been proposed as
an indicator of the biological impact of metals in natural
waters. Humic substances are composed of a wide variety of
molecules of many differing sizes with many ways to orient
themselves by twisting, bending, compressing and expanding.
They are loosely held together by weak forces in a colloidal
state. Changes in the ambient conditions (e.g. pH, ionic
strength, type of metals present, degree of metal loading)
can result in fragmentation and rapid rearrangement of the
humic colloids. As a result, humic substances have been
described as dynamic supermixtures of chemically and
physically heterogeneous components, whose metal binding
properties are influenced by environmental factors.
Knowledge about the nature of the interactions between humic
substances and metal ions is therefore essential for
improving our understanding of the mechanisms of that
determine the transport, and the fate and bioavailability of
trace metals in natural waters.
Metal species are
characterized by their physicochemical parameters, including
dissociation rate constants (kinetic reactivity), stability
constants (thermodynamic stability), diffusion coefficients
(mobility), free metal ion concentrations and ligand
concentrations. The techniques being employed to investigate
trace metal speciation in aqueous environmental samples
include equilibrium-based techniques, such as
Pseudopolarography; as well as kinetic-based techniques,
such as Diffusive Gradients in Thin Films (DGT) and
Competing Ligand Exchange Methods.
 |
 |
|
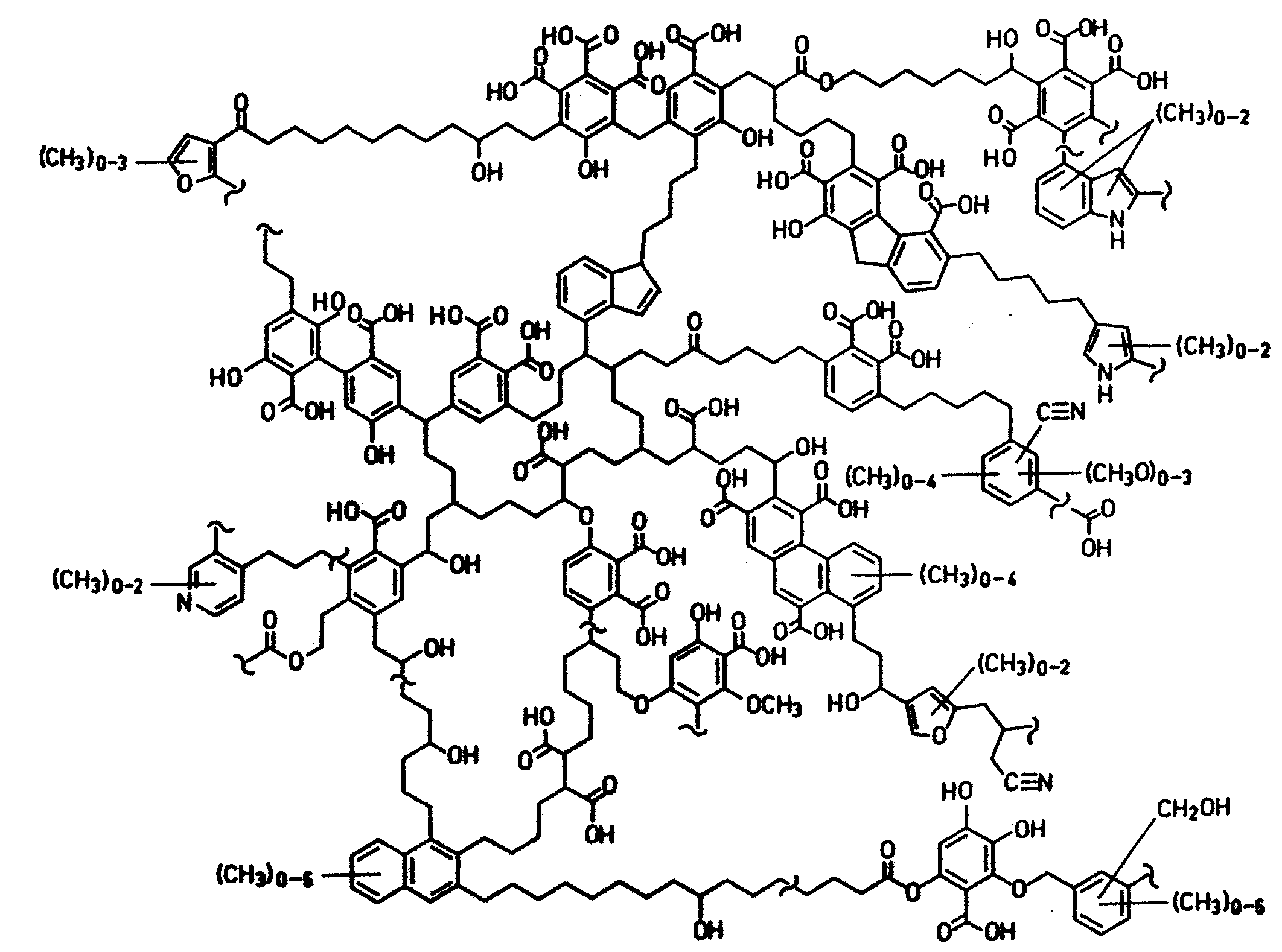
A proposed structure of a humic acid molecule (H.R.
Schulten, M. Schnitzer, Naturwissenschaften,
1993, 80, 27) showing a wide range of
functional groups (e.g. phelolic, carboxylic,
salicylic, phthalate, nitrogen-bearing groups)
that can bind to metal ions. |
The brown
colour of many freshwaters is due to the presence of humic
and fulvic acids, which help to moderate metal toxicity
through the complexation of metal pollutants. |
This research program is
directed at the development of sophisticated models and in
situ sample devices for the long-term monitoring and
interpretation of trace metal speciation and bioavailability
in the aquatic environment. The sampling devices will mimic
the diffusive layer at the biological membranes of aquatic
biota to provide an in situ, time-averaged estimate of
bioavailable metal concentrations. Development of in situ
approaches for multi-element speciation techniques in
natural waters constitutes an important advance towards
overcoming artifacts that plague laboratory-based techniques
such as contamination, analyte loss and sample
transformation during sample collection, handling and
storage.
The in situ sampling device
will consist of a four-layer system: 1) a metal binding
layer composed of a solid-phase ion exchange resin (e.g.
Chelex 100), 2) a porous membrane to hold the ion exchange
resin, 3) a well-defined, stagnant boundary layer (e.g.
deionized water) which controls mass transport to the
binding layer, and 4) a size-selective outer membrane for
size fractionation. Labile metals in the bulk solution
diffuse through the stagnant boundary layer and are
concentrated in the resin. Quantification of labile species
is important because lability can be correlated with metal
bioavailablility. The sampling device is based on the
coupled diffusion of the metal complex, ML, and the free
metal ion, M, from the sample medium through a stagnant
boundary layer, where only M is bound by the receiving
phase. This shifts the equilibrium between M and ML,
inducing a steady-state concentration gradient within the
boundary layer. ML is ‘labile’ when the formation and
dissociation kinetics are sufficiently fast that the total
metal flux towards the surface of the binding phase is
controlled exclusively by the coupled diffusion of M and ML.
Metal complexes not able to dissociate within the boundary
layer are ‘non-labile’. The thickness of the boundary layer
controls the analytical timescale of measurement; hence, it
is the critical parameter in defining the metal species that
are measured. Metal species are characterized by the
physicochemical parameters that define lability (and hence
bioavailability) at an interface: dissociation rate
constants (reactivity), stability constants (thermodynamic
stability), diffusion coefficients (mobility), and free
metal ion concentration. Labile metals concentrated by the
sampling device are eluted from the resin with 1 M nitric
acid. The metal concentrations in the eluate can be
determined by Graphite Furnace Atomic Absorption
Spectroscopy, Inductively Coupled Plasma – Mass
Spectrometry, or Anodic Stripping Voltammetry.
|

|
 |
|
A
steady-state
concentration gradient of ML is induced within
the boundary layer. Metal complexes are
classified as labile, quasi-labile or non-labile
depending on its ability to dissociate before
reaching the binding phase. |
An
array of Diffusive Gradients in Thin Film (DGT)
devices, which are similar in design to the
proposed sampling device. |
3. Analysis of air particulate matter by Scanning Electron
Microscopy
The widespread use of
radionuclides in a wide range of industries such as
petroleum engineering (well logging for oil exploration),
the airline industry (to check welds and structural
integrity) and medicine (cancer treatment and diagnostics)
has lead to concerns that these sources can be used to
create radiological dispersal devices (RDDs) for terrorist
attacks. Scanning Electron Microscopy and Energy Dispersive
X-ray Spectroscopy are being used to characterize the size,
shape, morphology and chemical composition of airborne
particulate matter from RDDs. Canada has initiated testing
on ceramic materials, focusing on SrTiO3, CaTiO3 and CeO2.
SrTiO3 is used to simulate Sr-90 used in Radioisotope
Thermoelectric Generators (a generator which obtains power
from radioactive decay), CaTiO3 is used to simulate SrTiO3
for outdoor testing, and CeO2 is used as a mechanical
surrogate for UO2, PuO2 & AmO2.
This micro-analytical approach
has the advantage of providing information about the behaviour and distribution of particulate material that
cannot be obtained by traditional bulk analytical
techniques. The threat from RDDs has been a topic of
considerable debate over the past few years. Expert opinions
on the risks from RDDs vary wildly. This research forms part
of a multidisciplinary counter terrorism program with
scientists from Defence Research and Development Canada,
Health Canada, the University of British Columbia and
Carleton University that will asses the risks from RDDs.
 |
 |
|
Ti element map for SrTiO3
particles |
EDS spectrum for
SrTiO3 particles |
|
 |
 |
|
Mottled SrTiO3 particle
|
Partially Melted
SrTiO3 particle |
|
 |
 |
|
Mottled CaTiO3
particle |
Wetted CeO2
particle |
|
 |
 |
|
Secondary Electron Image of
CeO2 and Si particles |
Backscatter Electron Image of
CeO2 and Si particles |
4. Mitigation of acidic waters: effects on the speciation of
aluminum
Aluminum is known to
contribute to fish mortality in lakes and rivers in Nova
Scotia. Aluminum toxicity is dependant not only on the total
dissolved Al concentration, but also on the Al speciation.
Inorganic monomeric aluminum species (e.g. Al3+, Al(OH)2+
and Al(OH)2+) are reported to be toxic, and these forms
predominate at low pH. Acid rain combined with low
alkalinity have resulted in acidic rivers and lakes in Nova
Scotia, thereby creating conditions that favour Al toxicity.
Current methods for mitigation of acidic freshwaters involve
addition of carbonate ions, usually done through the
addition of lime (CaCO3) using dosers (lime titrating
equipment). However, liming is expensive and must be applied
continually in order to remain effective.
The addition of a novel
carbonate-rich industrial waste product to acidic waters has
been proposed as being an inexpensive and potentially more
effective alternative to traditional liming practices, as
the material is abundant, and the carbonate ions are
available at twice the level of that in limestone. The
proposed approach involves investigating the effects of
introducing the new carbonate material into water samples
collected from the Felix Mill Brook (Claire, NS) on the pH
and aluminum speciation using ion exchange technique(s) as
well as Diffusive Gradients in Thin Films (DGT).
 |
|
Soil porewater samplers at the
Felix Mill Brook. |
5. Characterization of petroleum hydrocarbons in
contaminated soils
Contamination of soils by
petroleum hydrocarbons is a widespread environmental
problem. The source of such contamination is primarily
accidental release from gas stations and fuel depots.
Gasoline and diesel fuel have deleterious effects on plant
and animal life, and often leaches into local groundwater
supplies, posing a potential hazard to human health.
Contaminated soils can reduce the usability of land for
development, and weathered petroleum residuals may stay
bound to soils for years. The chemical composition of
petroleum products is complex and often subject to temporal
and spatial variations in the soil environment. Hence,
little is known about their potential for health or
environmental impacts. Total petroleum hydrocarbon
concentrations serve as gross measures of petroleum
contamination; however, more detailed information is
required to assess the risk to human and ecosystem health.
The objectives of this
research were to collect and analyze soils from six sites
located throughout the Maritimes that are potentially
contaminated with gasoline and/or diesel fuel. The study
areas are sites of decommissioned fuel depots and service
stations. Both field and laboratory analyses of hydrocarbon
contaminants were performed in order to determine their
concentrations and to identify the compounds present.
The Gastechor Hydrocarbon
Surveyor (“Sniffer”) was used to measure total hydrocarbon
concentrations in the soil based on catalytic combustion of
volatile species. It is easy to use and portable, making it
ideal for field measurements. For soils, hydrocarbon
concentrations in the headspace of enclosed samples are
directly proportional to concentrations in the soil. Solid
Phase Microextraction – Gas Chromatography – Mass
Spectrometry (SPME-GC-MS) was used to identify and quantify
the hydrocarbon compounds present in the soil samples. SPME
is a sensitive and relatively inexpensive sample preparation
technique developed by Janusz Pawlyszyn (Waterloo). It uses
non-exhaustive extraction to preconcentrate the sample in a
thin fibre, made up of an inner core of fused silica, and an
outer coating composed of a solid and/or liquid polymer
sorbent. A thin, stagnant layer develops adjacent to the
fibre, known as the boundary layer. Over time, the analyte
is concentrated in the fibre, so that at equilibrium, the
concentration in the fibre is greater than in the sample.
Following extraction of the analyte, the fibre is inserted
into a Gas Chromatograph (GC) injector port, and the sample
is thermally desorbed onto the GC column for analysis.
|
 |
 |
|
Digging a hole
|
Taking "Sniffer"
measurements |
|
 |
 |
|
Gasoline Chromatogram |
Diesel Chomatogram |
|

