| Home |
|
Part 1 Symmetry and Group Theory |
|
Part 2 Molecular Orbital Theory |
|
Part 3 Advanced MO Theory |
|
Part 4 Computational Chemistry |
|
Part 5 Main Group Chemistry |
| Resources |
Evaluating Lewis Structures
Question 1
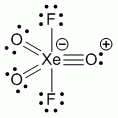
a. XeO3 (hybridisation: sp3)
Point Group = C3v
| C3v | E | 2C3 | 3σv |
| Γπ | 6 | 0 | 0 |
Γπ reduces to A1 + A2 + 2E
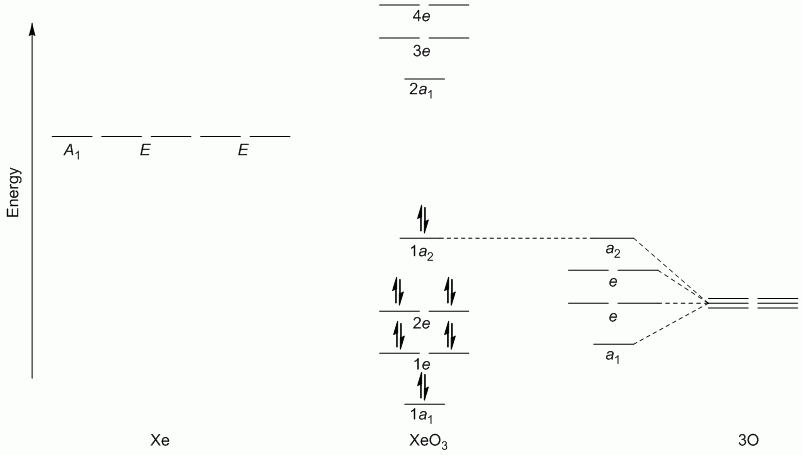
Lewis structure, assuming d orbitals are available for bonding:
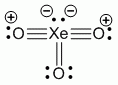
b. XeF2O3 (hybridisation: sp3d)
Point Group = D3h
| D3h | E | 2C3 | 3C2 | σh | 2S3 | 3σv |
| Γπ(O) | 6 | 0 | -2 | 0 | 0 | 0 |
Γπ(O) reduces to A2' + E' + 2A2" + E"
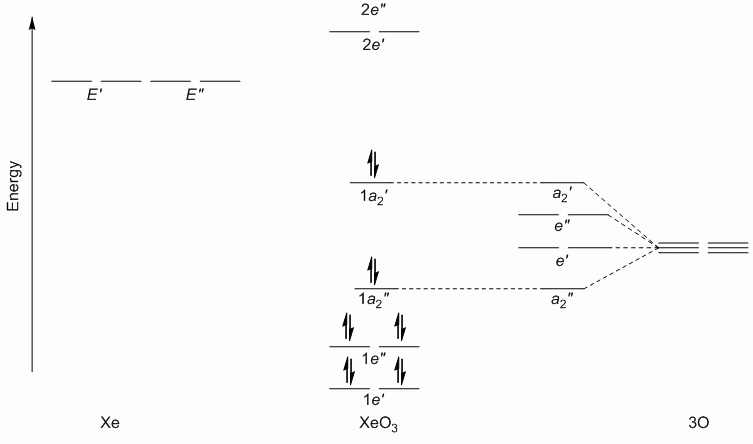
Lewis structure, assuming d orbitals are available for bonding: