| Home |
|
Part 1 Symmetry and Group Theory |
|
Part 2 Molecular Orbital Theory |
|
Part 3 Advanced MO Theory |
|
Part 4 Computational Chemistry |
|
Part 5 Main Group Chemistry |
| Resources |
Character Tables
Question 1
a. E
| 1 | 0 | 0 | ||
| 0 | 1 | 0 | ||
| 0 | 0 | 1 |
b. C2(x)
| 1 | 0 | 0 | ||
| 0 | -1 | 0 | ||
| 0 | 0 | -1 |
c. C3
| -1/2 | -√3/2 | 0 | ||
| √3/2 | -1/2 | 0 | ||
| 0 | 0 | 1 |
d. σh
| 1 | 0 | 0 | ||
| 0 | 1 | 0 | ||
| 0 | 0 | -1 |
e. C4
| 0 | -1 | 0 | ||
| 1 | 0 | 0 | ||
| 0 | 0 | 1 |
f. i
| -1 | 0 | 0 | ||
| 0 | -1 | 0 | ||
| 0 | 0 | -1 |
Question 2
| 1 | 0 | 0 | |||
| E = | 0 | 1 | 0 | ||
| 0 | 0 | 1 |
| -1/2 | -√3/2 | 0 | |||
| C3 = | √3/2 | -1/2 | 0 | ||
| 0 | 0 | 1 |
| -1 | 0 | 0 | |||
| C2 = | 0 | 1 | 0 | ||
| 0 | 0 | -1 |
| 1 | 0 | 0 | |||
| σh = | 0 | 1 | 0 | ||
| 0 | 0 | -1 |
| -1/2 | -√3/2 | 0 | |||
| S3 = | √3/2 | -1/2 | 0 | ||
| 0 | 0 | -1 |
| 1 | 0 | 0 | |||
| σv(xz) = | 0 | -1 | 0 | ||
| 0 | 0 | 1 |
Now block diagonalise the matricies. Since C3 and S3 have off-diagonal elements, each matrix must be divided into 2×2 and 1×1 matrices:
| 1 | 0 | 0 | |||||||
| E = | 0 | 1 | 0 | ||||||
| 0 | 0 | 1 | |||||||
| -1/2 | -√3/2 | 0 | |||||||
| C3 = | √3/2 | -1/2 | 0 | ||||||
| 0 | 0 | 1 | |||||||
| -1 | 0 | 0 | |||||||
| C2 = | 0 | 1 | 0 | ||||||
| 0 | 0 | -1 | |||||||
| 1 | 0 | 0 | |||||||
| σh = | 0 | 1 | 0 | ||||||
| 0 | 0 | -1 | |||||||
| -1/2 | -√3/2 | 0 | |||||||
| S3 = | √3/2 | -1/2 | 0 | ||||||
| 0 | 0 | -1 | |||||||
| 1 | 0 | 0 | |||||||
| σv(xz) = | 0 | -1 | 0 | ||||||
| 0 | 0 | 1 | |||||||
You now have the following after summing the 2x2 and 1x1 matrices:
| D3h | E | 2C3 | 3C2 | σh | 2S3 | 3σv | |
| 2 | -1 | 0 | 2 | -1 | 0 | (x,y) | |
| 1 | 1 | -1 | -1 | -1 | 1 | z |
Four rows are missing, since there needs to be the same number of rows as there are columns of operations, including the all symmetric row (Property 7):
| D3h | E | 2C3 | 3C2 | σh | 2S3 | 3σv | |
| 2 | -1 | 0 | 2 | -1 | 0 | (x,y) | |
| 1 | 1 | -1 | -1 | -1 | 1 | z | |
| 1 | 1 | 1 | 1 | 1 | 1 |
Three rows are still missing. The sum of the squares of the characters under E must equal the order of the group (Property 4), so:
22 + 12 + 12 + x2 + y2 + z2 = 12
x2 + y2 + z2 = 6
x = 2, y = 1, z =1
Irreducible representations are orthogonal to each other (Property 6). Starting with the x = 2 row, which we will start by assuming is simialr to the (x,y) row above, we could have either of the following two possibilities (the character under E is always positive):
| D3h | E | 2C3 | 3C2 | σh | 2S3 | 3σv | |
| 2 | -1 | 0 | 2 | -1 | 0 | (x,y) | |
| 1. | 2 | 1 | 0 | -2 | -1 | 0 | |
| 2. | 2 | -1 | 0 | -2 | 1 | 0 |
Both options are orthognal with the (x,y) row and the all symmetric row, but only the second combination is orthognal with the z row.
Two rows are still missing, each with a character of 1 under E. So they will all have characters of +/- 1. Using trial-and-error and some educated guessing (along with Property 6), you can solve for the missing rows. Aim for +6 and -6 when you add them up:
| D3h | E | 2C3 | 3C2 | σh | 2S3 | 3σv | |
| 2 | -1 | 0 | 2 | -1 | 0 | (x,y) | |
| 1 | 1 | -1 | -1 | -1 | 1 | z | |
| 1 | 1 | 1 | 1 | 1 | 1 | ||
| 2 | -1 | 0 | -2 | 1 | 0 | ||
| 1 | 1 | -1 | 1 | 1 | -1 | ||
| 1 | 1 | 1 | -1 | -1 | -1 |
Now add the symmetry labels (A, B, E, T) and any modifiers (1, 2, ', ", g, u)
| D3h | E | 2C3 | 3C2 | σh | 2S3 | 3σv | |
| E' | 2 | -1 | 0 | 2 | -1 | 0 | (x,y) |
| A2" | 1 | 1 | -1 | -1 | -1 | 1 | z |
| A1' | 1 | 1 | 1 | 1 | 1 | 1 | |
| E" | 2 | -1 | 0 | -2 | 1 | 0 | |
| A2' | 1 | 1 | -1 | 1 | 1 | -1 | |
| A1" | 1 | 1 | 1 | -1 | -1 | -1 |
Finally, by considering the symmetry of the d orbitals and rotations, add them to the character table:
| D3h | E | 2C3 | 3C2 | σh | 2S3 | 3σv | ||
| E' | 2 | -1 | 0 | 2 | -1 | 0 | (x,y) | (x2-y2,xy) |
| A2" | 1 | 1 | -1 | -1 | -1 | 1 | z | |
| A1' | 1 | 1 | 1 | 1 | 1 | 1 | z2 | |
| E" | 2 | -1 | 0 | -2 | 1 | 0 | (Rx,Ry) | (xz,yz) |
| A2' | 1 | 1 | -1 | 1 | 1 | -1 | Rz | |
| A1" | 1 | 1 | 1 | -1 | -1 | -1 |
Question 3
| -1/2 | -√3/2 | 0 | -1 | 0 | 0 | 1/2 | -√3/2 | 0 | |||||||||||||
| Missing Operation = C3(C2) = | √3/2 | -1/2 | 0 | 0 | -1 | 0 | = | √3/2 | 1/2 | 0 | = C6 | ||||||||||
| 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 |
Vibrational Spectroscopy
Question 1
a. SiCl4
3N = 15
3N-6 = 9
| Td | E | 8C3 | 3C2 | 6S4 | 6σd |
| Γtotal | 15 | 0 | -1 | -1 | 3 |
Reduces to: A1 + E + T1 + 3T2
| A1 | E | T1 | 3T2 | |
| Translations | (Tx,Ty,Tz) | |||
| Rotations | (Rx,Ry,Rz) | |||
| Vibrations | A1 | E | 2T2 | |
| IR Active | Yes | |||
| Raman Active | Yes | Yes | Yes |
IR = 2T2
Raman = A1 + E + 2T2
b. trans-SF2Cl4
3N = 21
3N-6 = 15
| D4h | E | 2C4 | C2 | 2C2' | 2C2" | i | 2S4 | σh | 2σv | 2σd |
| Γtotal | 21 | 3 | -3 | -3 | -1 | -3 | -1 | 5 | 5 | 3 |
Reduces to: 2A1g + A2g + B1g + B2g + 2Eg + 3A2u + B2u + 4Eu
| 2A1g | A2g | B1g | B2g | 2Eg | 3A2u | B2u | 4Eu | |
| Translations | Tz | (Tx,Ty) | ||||||
| Rotations | Rz | (Rx,Ry) | ||||||
| Vibrations | 2A1g | B1g | B2g | Eg | 2A2u | B2u | 3Eu | |
| IR Active | Yes | Yes | ||||||
| Raman Active | Yes | Yes | Yes | Yes |
IR = 2A2u + 3Eu
Raman = 2A1g + B1g + B2g + Eg
c. SbCl5
3N = 18
3N-6 = 12
| D3h | E | 2C3 | 3C2 | σh | 2S3 | 3σv |
| Γtotal | 18 | 0 | -2 | 4 | -2 | 4 |
Reduces to: 2A1' + A2' + 4E' + 3A2" + 2E"
| 2A1' | A2' | 4E' | 3A2" | 2E" | |
| Translations | (Tx,Ty) | Tz | |||
| Rotations | Rz | (Rx,Ry) | |||
| Vibrations | 2A1' | 3E' | 2A2" | E" | |
| IR Active | Yes | Yes | |||
| Raman Active | Yes | Yes | Yes |
IR = 3E' + 2A2"
Raman = 2A1' + 3E' + E"
d. CH2Cl2
3N = 15
3N-6 = 9
| C2v | E | C2 | σv | σv' |
| Γtotal | 15 | -1 | 3 | 3 |
Reduces to: 5A1 + 2A2 + 4B1 + 4B2
| 5A1 | 2A2 | 4B1 | 4B2 | |
| Translations | Tz | Tx | Ty | |
| Rotations | Rz | Ry | Rx | |
| Vibrations | 4A1 | A2 | 2B1 | 2B2 |
| IR Active | Yes | Yes | Yes | |
| Raman Active | Yes | Yes | Yes | Yes |
IR = 4A1 + 2B1 + 2B2
Raman = 4A1 + A2 + 2B1 + 2B2
Resonance Spectroscopy
Question 1
a. PF6-
19F NMR:
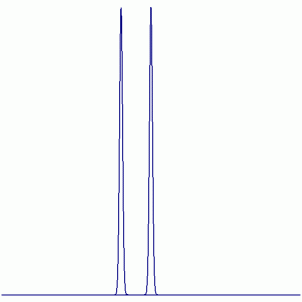
Peak Height Ratio: 1 : 1
Explaination: All F atoms are equivalent (i.e. exchangable by symmetry operations) and couple with one P atom (31P: I = ½, 100% abundant).
b. cis-195PtF2Cl22-
19F NMR:

Peak Height Ratio: 1 : 1
Explaination: Both F atoms are equivalent (i.e. exchangable by symmetry operations) and couple with one Pt atom that is only the Pt-195 isotope (195Pt: I = ½).
c. fac-MoF3(PMe3)3
19F NMR:
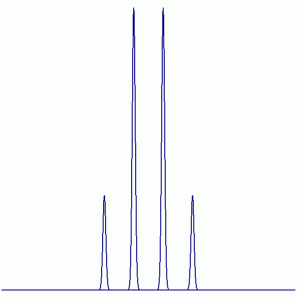
Peak Height Ratio: 1 : 3 : 3 : 1
Explaination: The three F atoms are equivalent (i.e. exchangable by symmetry operations) and couple with the three equivalent P atoms (31P: I = ½, 100% abundant).
d. DF
19F NMR:
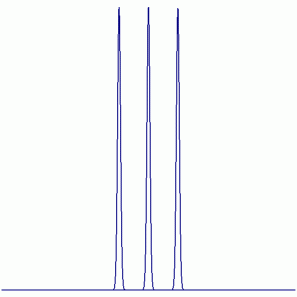
Peak Height Ratio: 1 : 1 : 1
Explaination: The F atom couples with the D atom (D = 2H: I = 1)
e. SiFCl3
19F NMR:
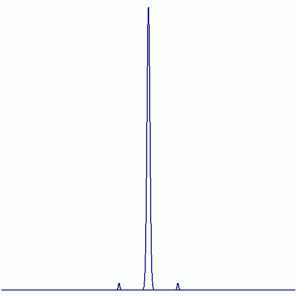
Peak Height Ratio: 2.35 : 95.3 : 2.35
Explaination: The F atom couples with 29Si when present (resulting in satellites 4.7% of the time), the other isotopes of silicon are not NMR active (29Si: I = ½, 4.7% abundant)
f. SeF4
19F NMR:
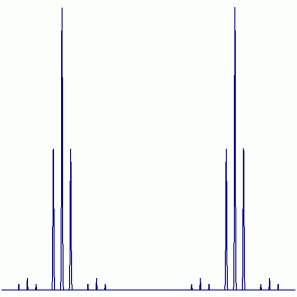
Peak Height Ratio: 3.8 : 7.6 : 3.8 : 92.4 : 184.8 : 92.4 : 3.8 : 7.6 : 3.8
Explaination: The are two unique sets of F atoms. Each set will couple with the Se 7.2% of the time and couple with the other set of two F atoms always (77Se: I = ½, 7.6% abundant; 19F: I = ½, 100% abundant).
g. mer-PF3Cl3-
19F NMR:
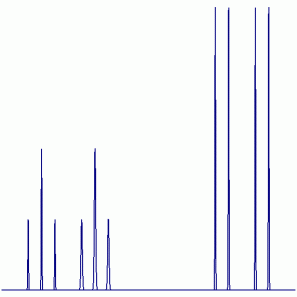
Peak Height Ratio: 1 : 2 : 1 : 1 : 2 : 1 : 4 : 4 : 4 : 4
Explaination: Two of the three F atoms are equivalent (i.e. exchangable by symmetry operations), while the third atom is unique. The F atoms will couple with the P atoms and the non-equivalent F atoms (31P: I = ½, 100% abundant; 19F: I = ½, 100% abundant). The signal for the unique F atom is one the left and the signal for the two equivalent F atoms is on the right.
h. 57FeF4-
19F NMR:

Peak Height Ratio: 1 : 1
Explaination: All F atoms are equivalent (i.e. exchangable by symmetry operations) and couple with one Fe atom that is only the Fe-57 isotope (57Fe: I = ½).
i. AgFPMe3
19F NMR:
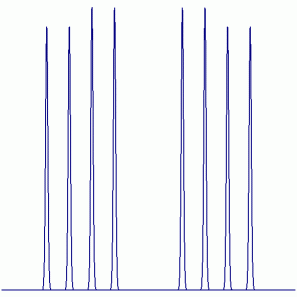
Peak Height Ratio: 24.1 : 24.1 : 25.9 : 25.9 : 25.9 : 25.9 : 24.1 : 24.1
Explaination: The F atom couples to the Ag atom, which has two NMR active isotopes (107Ag: I = ½, 51.8% abundant; 109Ag: I = ½, 48.2% abundant) and then couples with the P atom (31P: I = ½, 100% abundant).
j. AlF4-
19F NMR:
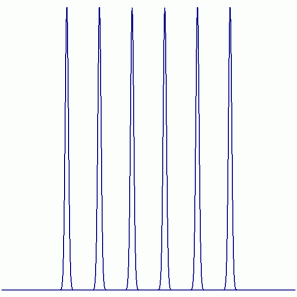
Peak Height Ratio: 1 : 1 : 1 : 1 : 1 : 1
Explaination: All F atoms are equivalent (i.e. exchangable by symmetry operations) and couple with one Al atom (27Al: I = 5/2, 100% abundant).