| Home |
|
Part 1 Atomic Structure |
|
Part 2 Simple Bonding Theory |
|
Part 3 Symmetry and Group Theory |
|
Part 4 Molecular Orbital Theory |
|
Part 5 Coordination Chemistry |
Coordination Complexes and Molecular Formulas
Question 1
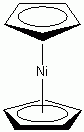
a. η5-Cp2Ni
b. fac-CuF3(H2O)3–

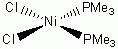
c. cis-NiCl2(PMe3)2
d. CoCl2Br2–
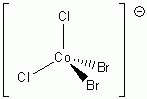
e. μ-Cl2-(Cu(dppe))2
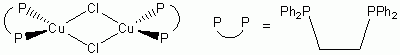
d Orbital Splitting Diagrams
Question 1
a. Octahedral
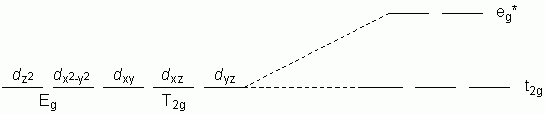
b. Octahedral with π-acceptor ligands
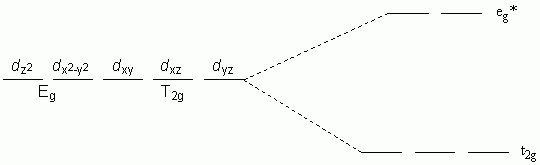
c. Octahedral with π-donor ligands
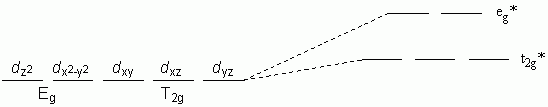
d. Tetrahedral
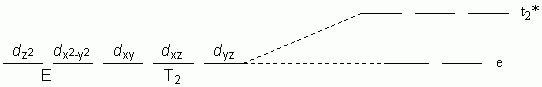
e. Square Planar
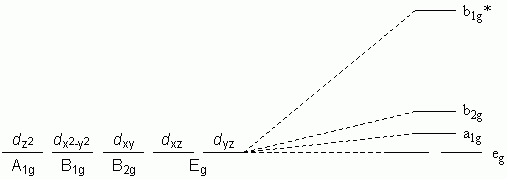
f. Trigonal Bipyramidal

g. Square-based Pyramid with axial-base angle = 90 °
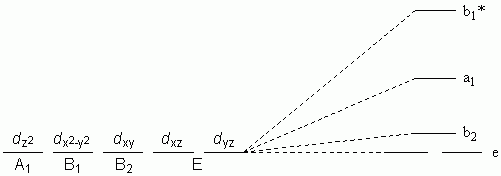
h. Square-based Pyramid with axial-base angle > 90 °
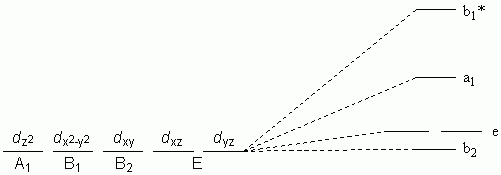
Ligand Field Stabilisation Energy (LFSE)
Question 1
a. d3
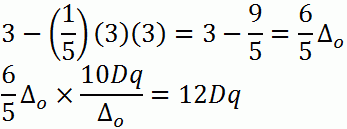
b. d5 (high-spin)
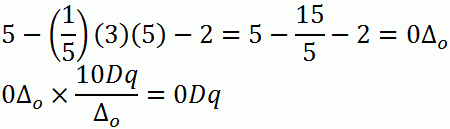
c. d5 (low-spin)
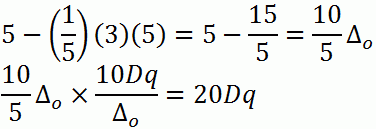
d. d9
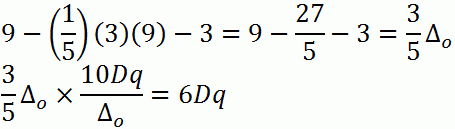
Question 2
a. d2
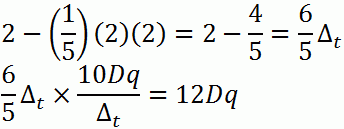
b. d4
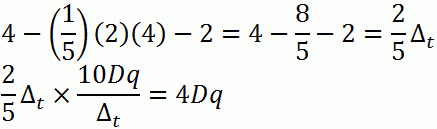
c. d7
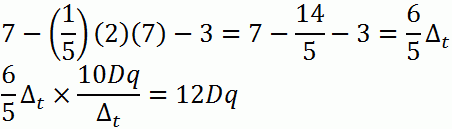
d. d10
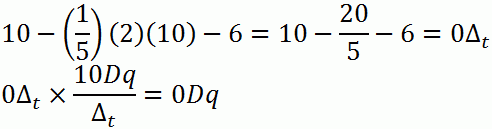
Coordination Complexes and Colour
Question 1
CrO3 is d0, therefore the colour is not due to d→d transitions. Since Cr is in a high oxidation state (+6) and attached to electron-rich oxygen atoms, the colour is caused by ligand-to-metal charge transfer (LMCT). The colour is dark because LMCT is not restricted by any selection rules.
Question 2
PCy3 is a stronger σ donor and π acceptor than PPh3, which results in the PCy3 complex to be square planar and the PPh3 complex to be tetrahedral. The d→d transtion is higher in energy for a square planar complex compared to a tetrahedral because the dx2-y2 orbital used in the square planar geometry lies along the bond axis, resulting in the anti-bonding b1g to be high in energy compared to the antibonding orbitals in a tetrahedron that result from poor orbital overlap (and are therefore lower) in energy. If red is observed (square planar), then green light is absorbed amd if green is observed (tetrahedral) then red light is absorbed. Green light is higher energy than red light, corresponding to the energy differences between Δsp and Δt.
Question 3
Cl and H2O are weak field ligands, which will result in the Mn complex to be high-spin d5. All d orbitals will be occupied by 1 unpaired electron, therefore the transitions are forbidden because ΔS = 0 (there can be no spin flips). With six of the same ligands and all d orbitals equally occupied, the molecular geometry will be a perfect octahedron, so transiitons will only occur during vibrational distortions. Both of these factors will result in pale colour.
CO is a strong field ligand, which will result in the Mn complex to be low-spin d6. The t2g orbitals will each be occupied by 2 electrons, so electrons can jump to eg* without flipping their spins. Since there are 5 CO ligands and 1 Br ligand, the complex will be permanently distorted from Oh symmetry, and so there will be a permanent mixing of p and d orbitals. Both of these factors will result in an intense colour.