octahedral geometry

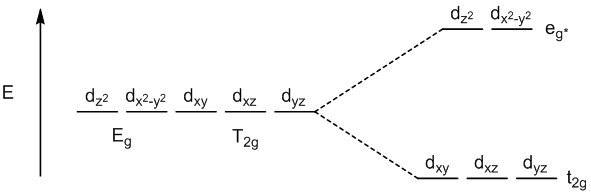
octahedral geometry with π-acceptor ligands
octahedral geometry with π-donnor ligands

tetrahedral geometry
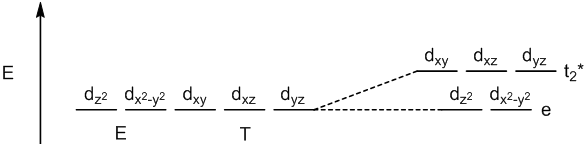
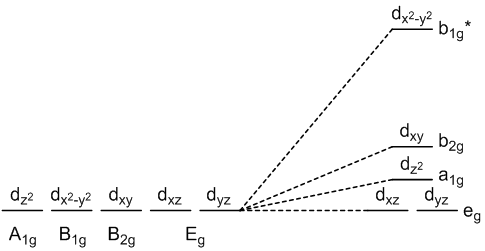
square planar geometry
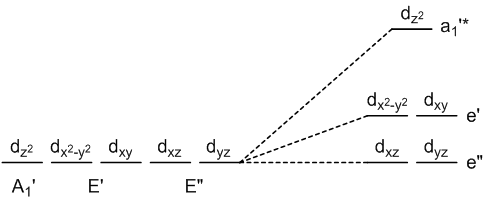
trigonal bipyramidal geometry
square pyramidal geometry (axial-base angle = 90°)
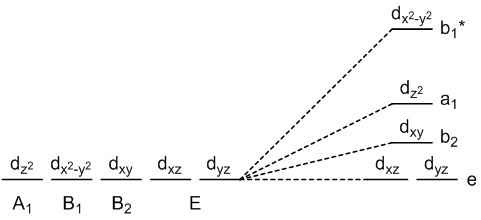
square pyramidal geometry (axial-base angle > 90°)
