| Home |
|
Part 1 Atomic Structure |
|
Part 2 Simple Bonding Theory |
|
Part 3 Symmetry and Group Theory |
|
Part 4 Molecular Orbital Theory |
|
Part 5 Coordination Chemistry |
Symmetry Adapted Linear Combinations (SALCs)
Question 1
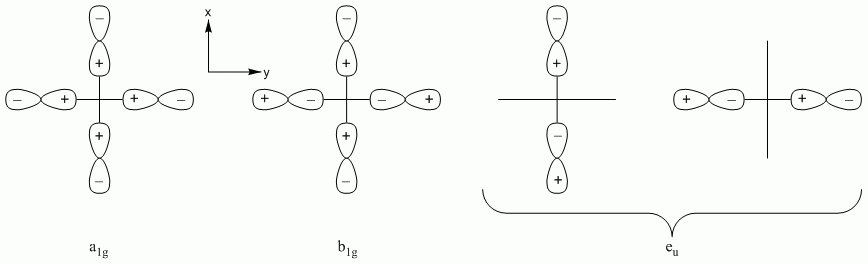
a. PdCl42- (square planar)
Point Group = D4h
| D4h | E | 2C4 | C2 | 2C2' | 2C2" | i | 2S4 | σh | 2σv | 2σd |
| Γσ | 4 | 0 | 0 | 2 | 0 | 0 | 0 | 4 | 2 | 0 |
Reduces to A1g + B1g + Eu
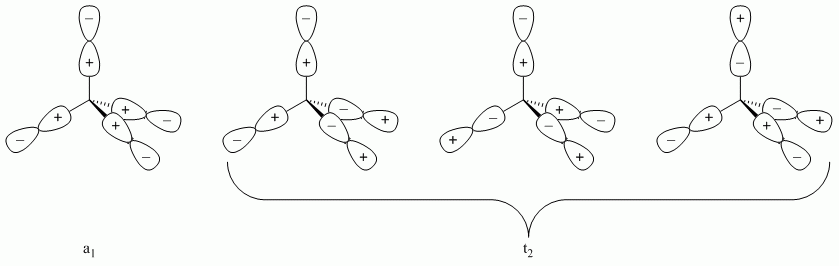
b. SiCl4
Point Group = Td
| Td | E | 8C3 | 3C2 | 6S4 | 6σd |
| Γσ | 4 | 1 | 0 | 0 | 2 |
Reduces to A1 + T2
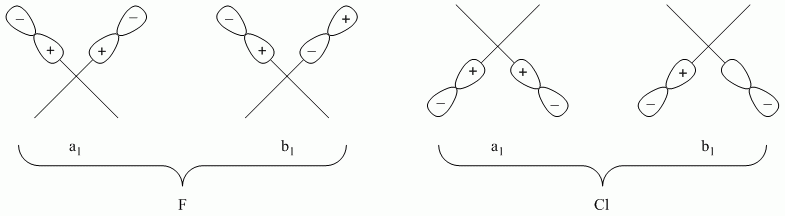
c. cis-PtF2Cl22-
Point Group = C2v
| C2v | E | C2 | σv | σv' |
| Γσ(F) | 2 | 0 | 2 | 0 |
| Γσ(Cl) | 2 | 0 | 2 | 0 |
Reduces to A1 + B1 for both F and Cl
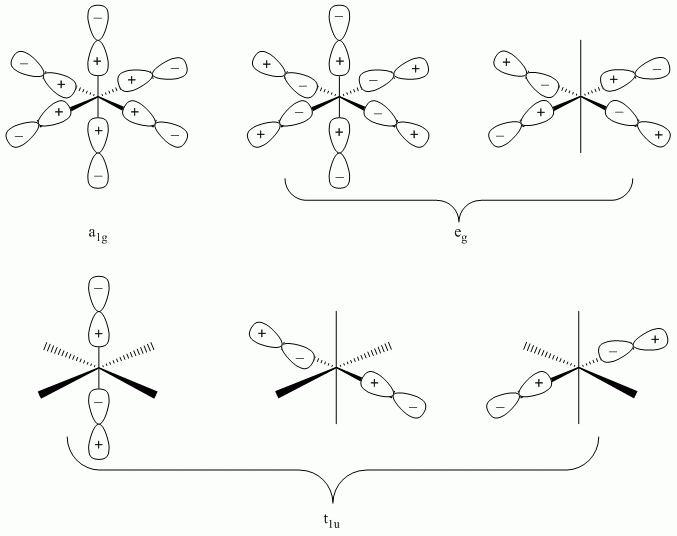
d. SF6
Point Group = Oh
| Oh | E | 8C3 | 6C2 | 6C4 | 3C2 | i | 6S4 | 8S6 | 3σh | 6σd |
| Γσ | 6 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 4 | 2 |
Reduces to A1g + Eg + T1u
Question 2
a. PdCl42- (square planar)
b. SiCl4
c. cis-PtF2Cl22-
d. SF6
Molecular Orbital Diagrams (σ-bonding)
Question 1
a. trans-SF2Cl4
Point Group = D4h
| D4h | E | 2C4 | C2 | 2C2' | 2C2" | i | 2S4 | σh | 2σv | 2σd |
| Γσ(F) | 2 | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 2 |
| Γσ(Cl) | 4 | 0 | 0 | 2 | 0 | 0 | 0 | 4 | 2 | 0 |
Γσ(F) reduces to A1g + A2u
Γσ(Cl) reduces to A1g + B1g + Eu
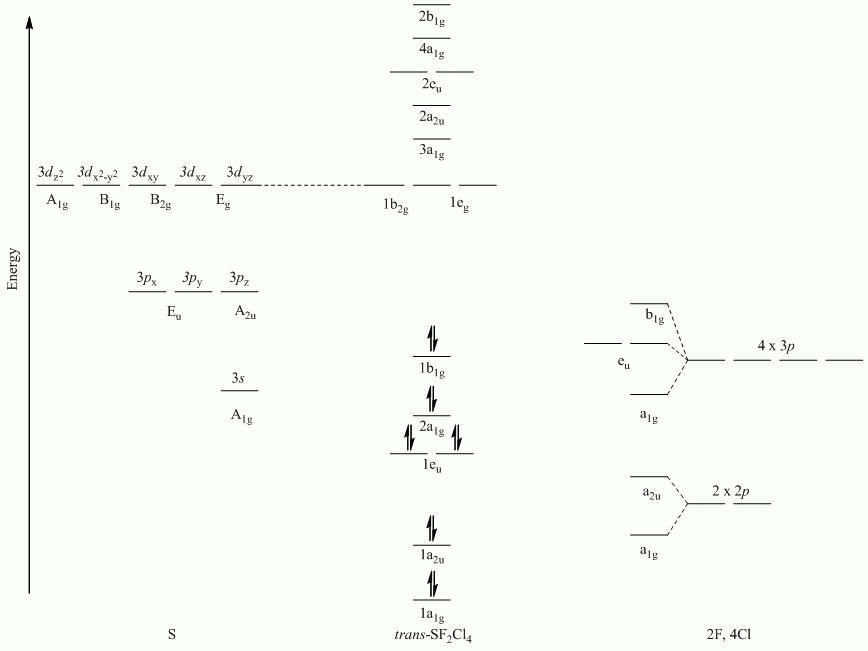
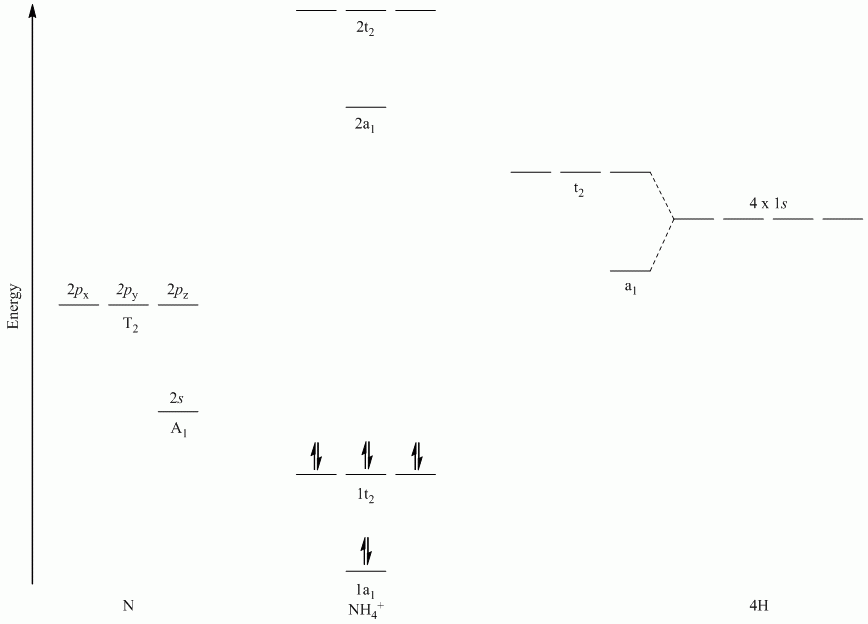
b. NH4+
Point Group = Td
| Td | E | 8C3 | 3C2 | 6S4 | 6σd |
| Γσ | 4 | 1 | 0 | 0 | 2 |
Reduces to A1 + T2
c. SF6
Point Group = Oh
| Oh | E | 8C3 | 6C2 | 6C4 | 3C2 | i | 6S4 | 8S6 | 3σh | 6σd |
| Γσ | 6 | 0 | 0 | 2 | 2 | 0 | 0 | 0 | 4 | 2 |
Reduces to A1g + Eg + T1u
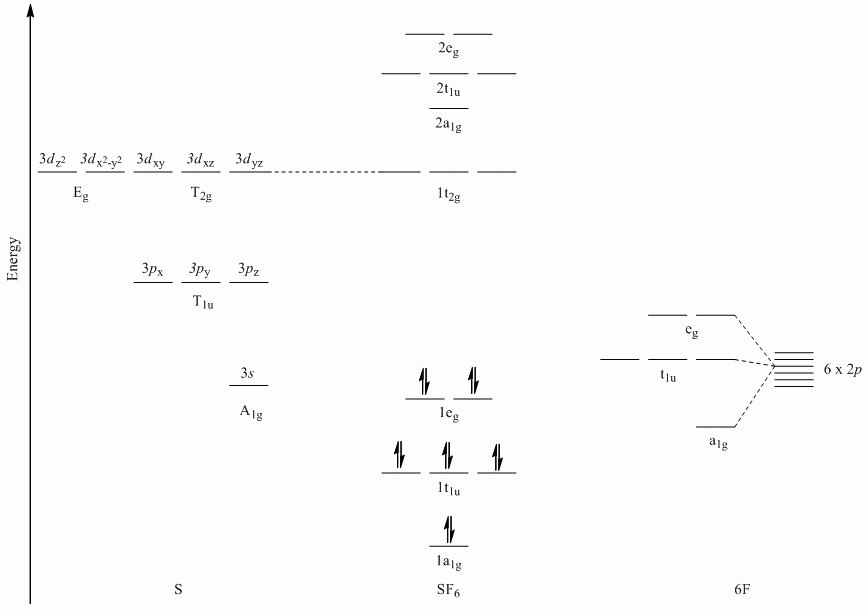
Molecular Orbital Diagrams (π-bonding)
Question 1
a. SiF4
Point Group = Td
All sets of vectors lie along a C3 rotation axis, therefore the p orbitals cannot be separated into subsets
| Td | E | 8C3 | 3C2 | 6S4 | 6σd |
| Γπ | 8 | -1 | 0 | 0 | 0 |
Reduces to E + T1 + T2
Note: Si used s, px, py and pz orbitals for σ-bonding
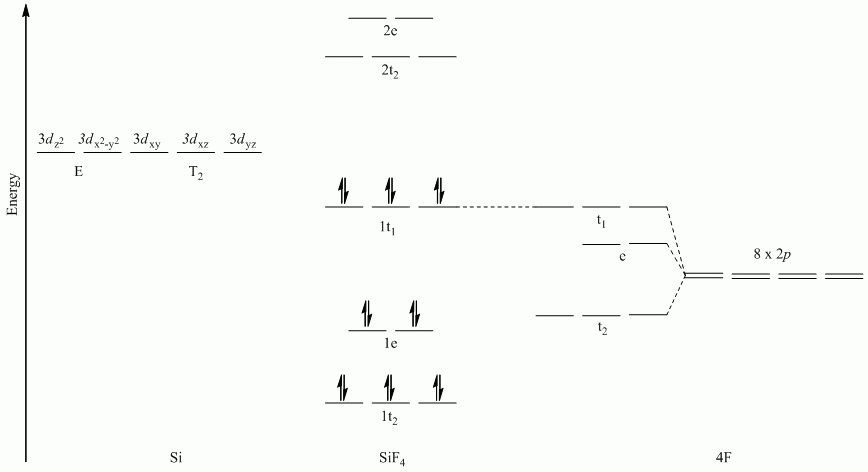
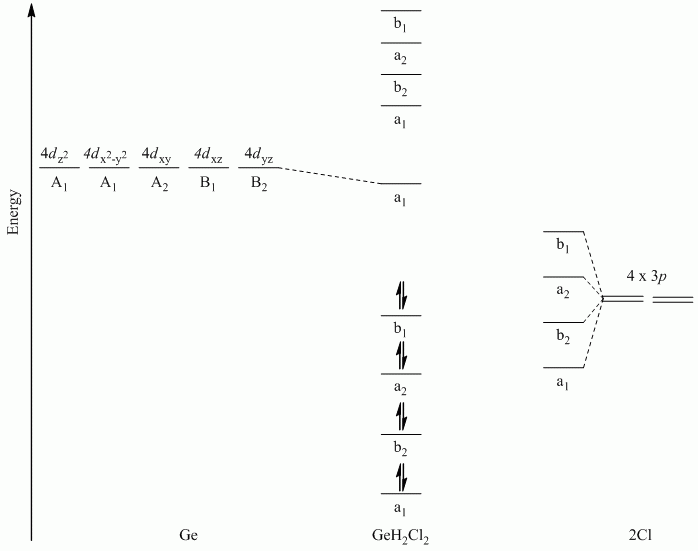
b. GeH2Cl2
Point Group = C2v
Only the Cl atoms can participate in π-bonding. The Cl atoms lie along a C1 rotation axis, therefore the p orbitals can be separated into perpendicular and parallel to the Cl-Ge-Cl plane.
| C2v | E | C2 | σv | σv' |
| Γπ⊥ | 2 | 0 | -2 | 0 |
| Γπ // | 2 | 0 | 2 | 0 |
Γπ⊥ reduces to A2 + B2
Γπ // reduces to A1 + B1
Hint: Draw the SALCs to determine the relative order
Note: Ge used s, px, py and pz orbitals for σ-bonding
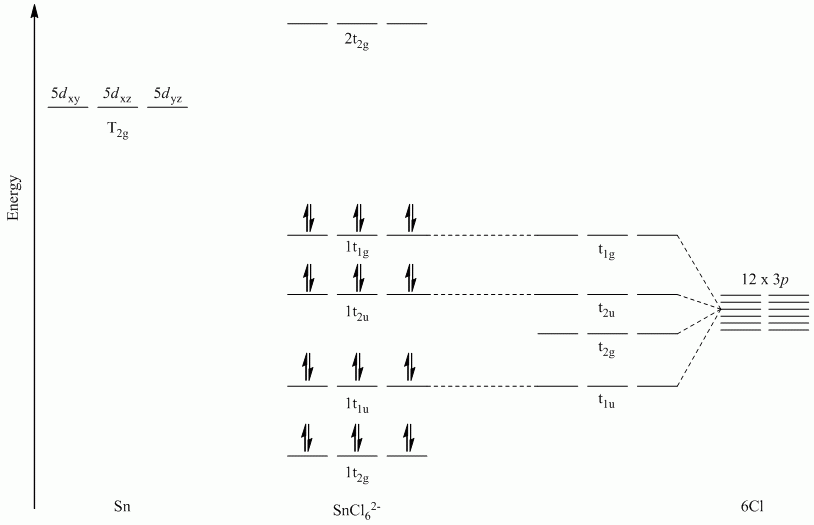
c. SnCl62-
Point Group = Oh
All sets of vectors lie along a C4 rotation axis, therefore the p orbitals cannot be separated into subsets
| Oh | E | 8C3 | 6C2 | 6C4 | 3C2 | i | 6S4 | 8S6 | 3σh | 6σd |
| Γσ | 12 | 0 | 0 | 0 | -4 | 0 | 0 | 0 | 0 | 0 |
Reduces to T1g + T2g + T1u + T2u
Question 2
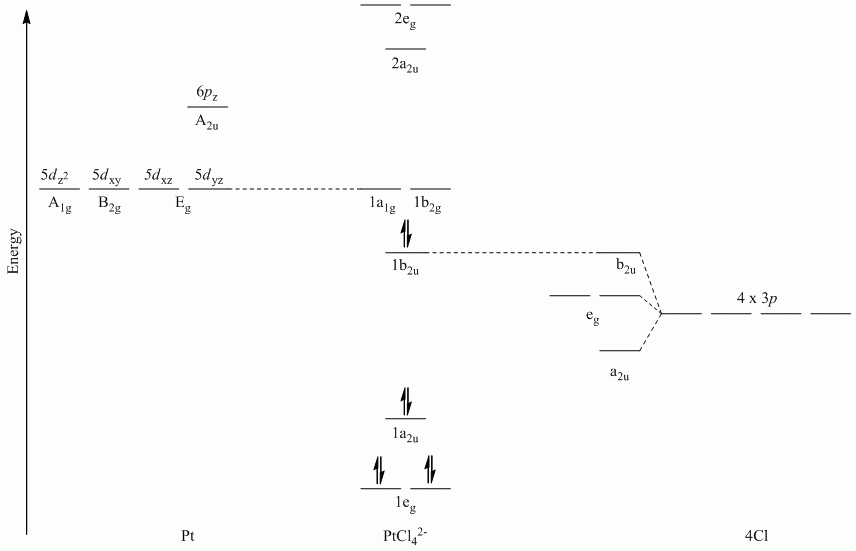
a. π-bonding perpendicular to the plane
Point Group = D4h
The vectors lie along a C2 rotation axis, there the p orbitals can be separated into one set of four perpendicular to the plane and one set of four parallel to the plane.
Recall that d block elements use ns, np and (n-1)d orbitals for bonding, and that (n-1)d orbitals are lower in energy than ns and np orbitals.
| D4h | E | 2C4 | C2 | 2C2' | 2C2" | i | 2S4 | σh | 2σv | 2σd |
| Γπ⊥ | 4 | 0 | 0 | -2 | 0 | 0 | 0 | -4 | 2 | 0 |
Reduces to Eg + A2u + B2u
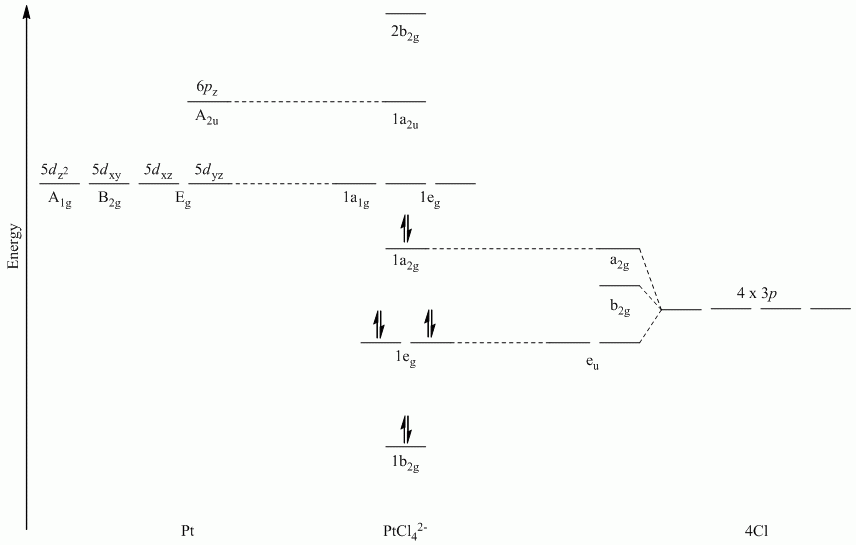
b. π-bonding parallel to the plane
Point Group = D4h
The vectors lie along a C2 rotation axis, there the p orbitals can be separated into one set of four perpendicular to the plane and one set of four parallel to the plane.
Recall that d block elements use ns, np and (n-1)d orbitals for bonding, and that (n-1)d orbitals are lower in energy than ns and np orbitals.
| D4h | E | 2C4 | C2 | 2C2' | 2C2" | i | 2S4 | σh | 2σv | 2σd |
| Γπ⊥ | 4 | 0 | 0 | -2 | 0 | 0 | 0 | 4 | -2 | 0 |
Reduces to A2g + B2g + Eu
Molecular Orbital Diagrams (π-bonding in Planar Molecules)
Question 1
a. C4H4
Point Group = D4h
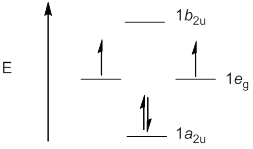
b. B3N3H6 (Borazine)
Point Group = D3h
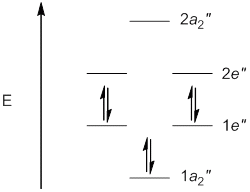
B3N3H6 is aromatic.
c. C8H82-
Point Group = D8h
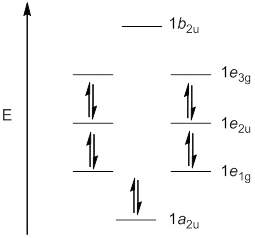
C8H82- is aromatic.